Ozempic Explained: The Science, Benefits, Risks, and Future of Weight-Loss Drugs
Obesity has long been framed as a failure of discipline rather than a chronic disease. This framing is now being dismantled by a growing body of neuroscientific, endocrinological, and metabolic research. At the center of this shift is Ozempic (semaglutide) and a new generation of GLP-1 receptor agonists, which have demonstrated weight-loss outcomes previously seen only with bariatric surgery.
Originally approved for type 2 diabetes, Ozempic has rapidly entered public consciousness as a powerful weight-loss intervention—celebrated, criticized, and debated in equal measure. But beyond celebrity headlines and social media hype lies a robust scientific story rooted in gut-brain signaling, appetite regulation, and hormonal control.
Glucagon-like peptide-1 (GLP-1) receptor agonists have transformed the treatment landscape for obesity and type 2 diabetes. Originally developed for glycemic control, drugs like semaglutide (marketed as Ozempic for diabetes and Wegovy for weight management) have gained prominence for their profound weight-loss effects. These medications mimic the GLP-1 hormone, which regulates appetite, insulin secretion, and gastric emptying.
What Is Ozempic?
Ozempic is the brand name for semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist originally developed to treat type 2 diabetes. It mimics the action of endogenous GLP-1, a hormone released after meals that enhances insulin secretion and regulates appetite. While Ozempic is approved for diabetes management, its higher-dose formulation (Wegovy) is FDA-approved for chronic weight management. Its widespread off-label use reflects both its efficacy and the unmet need for effective treatments for obesity.
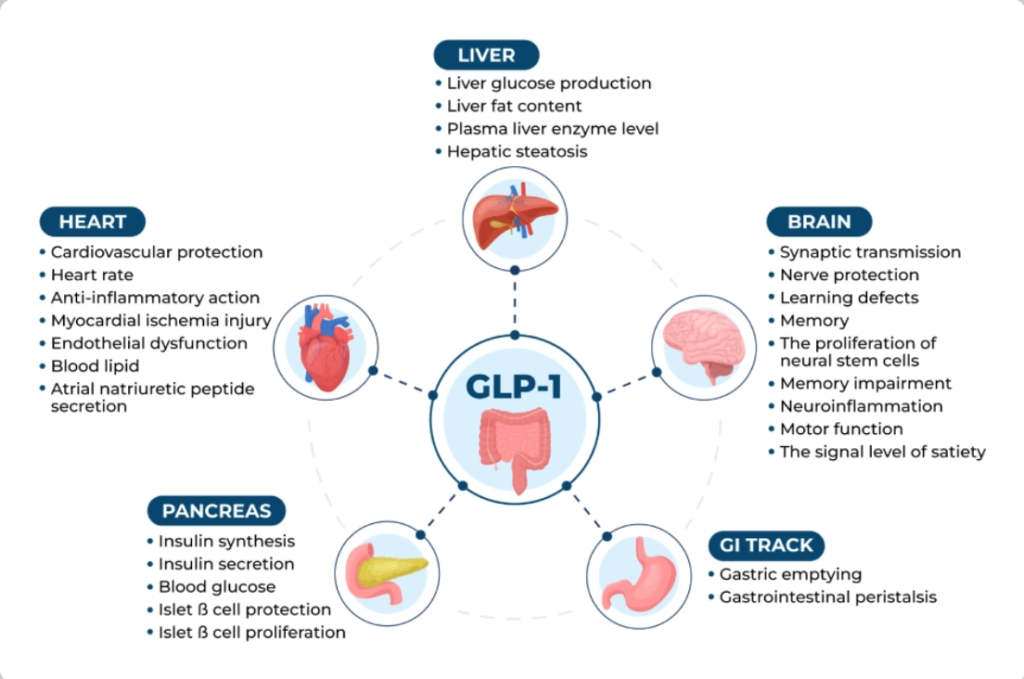
GLP-1 is an incretin hormone released from intestinal L-cells in response to nutrient intake. It enhances glucose-dependent insulin secretion, suppresses glucagon, delays gastric emptying, and promotes satiety via central nervous system pathways.
GLP-1 agonists bind to GLP-1 receptors, amplifying these effects:
- Central effects: Reduce appetite and food intake by acting on hypothalamic and brainstem regions.
- Peripheral effects: Slow gastric emptying, improve insulin sensitivity, and reduce postprandial glucose excursions.
- Additional benefits: Anti-inflammatory actions, improved lipid profiles, and potential cardioprotection.
Long-acting agonists like semaglutide have extended half-lives, enabling once-weekly dosing.
Clinical Efficacy in Weight Loss: The STEP Trials for Semaglutide
The Semaglutide Treatment Effect in People with obesity (STEP) program comprised multiple phase 3 RCTs evaluating once-weekly subcutaneous semaglutide 2.4 mg in adults with overweight or obesity.
Key findings from STEP 1-5 trials:
- STEP 1 (NEJM, 2021): In 1,961 non-diabetic participants, semaglutide led to -14.9% mean weight loss vs. -2.4% placebo at 68 weeks (difference: -12.4%). 86% achieved ≥5% loss, 70% ≥10%, 50% ≥15%.
- STEP 2 (patients with type 2 diabetes): -9.6% loss with 2.4 mg vs. -3.4% placebo.
- STEP 3 (with intensive behavioral therapy): -16% loss.
- STEP 4 (withdrawal study): Continuing semaglutide maintained loss; switching to placebo led to regain.
- STEP 5 (104 weeks): Sustained -15.2% loss vs. -2.6% placebo.
Meta-analyses confirm ~12-15% average loss in non-diabetics, superior to prior agents.

Citation
https://www.nejm.org/doi/full/10.1056/NEJMoa2032183
Comparison with Other Weight-Loss Drugs
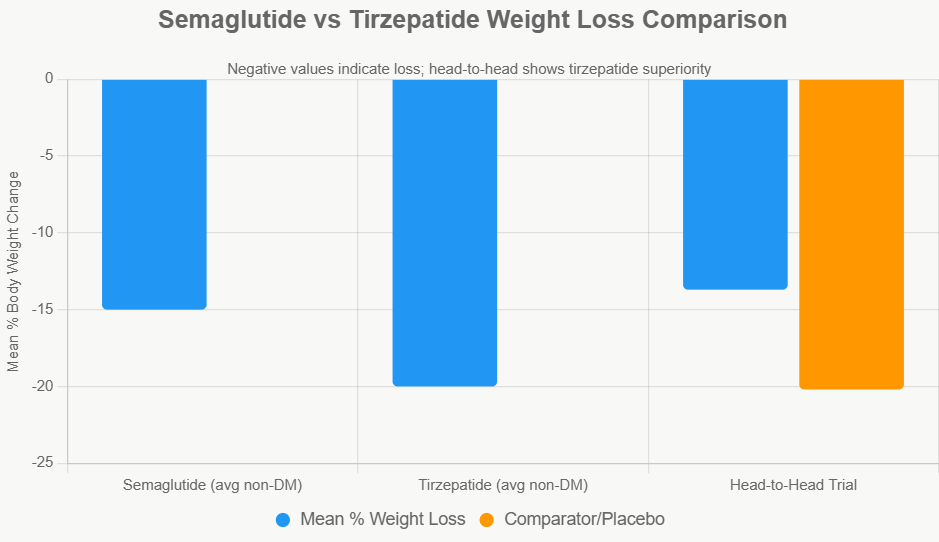
Compared with earlier GLP-1 drugs such as liraglutide, semaglutide demonstrates superior weight-loss efficacy, while newer dual agonists like tirzepatide show even greater reductions. Tirzepatide activates both GLP-1 and GIP receptors, resulting in weight losses exceeding 20% in some trials. These findings suggest that multi-hormonal targeting may represent the next evolution in obesity pharmacotherapy.
Comparison with Tirzepatide: Dual Agonist Advantage
Tirzepatide (Mounjaro/Zepbound) is a dual GLP-1/GIP receptor agonist, showing superior weight loss.
- SURMOUNT trials: ~15-21% loss.
- Head-to-head (NEJM, 2025): Tirzepatide -20.2% vs. semaglutide -13.7% at 72 weeks in non-diabetics.
- Real-world data: Greater likelihood of ≥15% loss with tirzepatide.
Tirzepatide often outperforms semaglutide by 4-7% in direct comparisons.
Beyond Weight Loss: Metabolic and Cardiovascular Benefits
Documented Benefits
Studies report:
- Reduced HbA1c
- Improved insulin sensitivity
- Lower systolic blood pressure
- Reduced LDL cholesterol
- Decreased inflammatory markers
Cardiovascular Outcomes
The SELECT trial (2023) showed:
- 20% reduction in major adverse cardiovascular events
- Even in non-diabetic obese patients
(American Heart Association presentation, 2023)
Side Effects and Medical Risks
The most common side effects of semaglutide involve the gastrointestinal system, including nausea, vomiting, and constipation, particularly during dose escalation. Rare but serious risks such as pancreatitis and gallbladder disease have been reported, and rodent studies suggesting thyroid tumors have led to precautionary warnings. Ongoing pharmacovigilance studies continue to assess long-term safety in large populations.
Common adverse events are gastrointestinal (GI):
- Nausea (30-50%), vomiting, diarrhea, constipation.
- Mostly mild-moderate, transient during dose escalation.
- Discontinuation rates: 5-10% due to GI issues.
Serious risks:
- Pancreatitis (rare, no increased risk in meta-analyses).
- Gallbladder events (slightly higher).
- Thyroid C-cell tumors (rodent data; contraindicated in MTC history).
No increased suicidality or major psychiatric risks per recent reviews.
Long-term safety from CVOTs (e.g., SELECT) shows favorable profile.
Ethical and Public Health Considerations
The rapid popularity of Ozempic has sparked ethical debates around access, equity, and prioritisation, particularly during drug shortages affecting diabetic patients. Public health experts argue that while pharmacological treatment is valuable, it must complement—not replace—broader strategies addressing food environments, socioeconomic inequality, and preventive care. Public health ethicists argue that in systems with finite resources, prioritising demand driven by aesthetic or lifestyle goals over chronic disease management risks widening health inequities, particularly for lower-income and marginalised populations who already face barriers to obesity and diabetes care.
Another ethical dimension concerns lifelong dependency and informed consent. Since evidence shows significant weight regain after discontinuation, many patients may require indefinite treatment to maintain benefits. This raises questions about long-term safety data, cost sustainability, and whether patients fully understand that these drugs function more like chronic disease therapies than short-term interventions. Public health researchers emphasise the need for transparent counselling, regulatory oversight, and post-marketing surveillance to ensure that enthusiasm does not outpace evidence, particularly as newer, more potent agents enter the market.
From a population-health perspective, experts increasingly argue for a hybrid model: one that integrates pharmacotherapy with behavioural support, mental-health care, and structural reform. Used responsibly, GLP-1 drugs could reduce obesity-related morbidity, cardiovascular disease, and healthcare costs at scale. Used irresponsibly, they risk becoming another high-cost intervention that benefits a narrow segment while leaving underlying social drivers untouched. The ethical challenge, therefore, lies not in the drugs themselves, but in how societies choose to deploy them.
Citation
https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
Psychological and Behavioral Effects
Patients frequently report a reduction in “food noise”—the constant mental preoccupation with eating—while on Ozempic. This effect appears to be mediated by changes in brain reward processing rather than conscious restraint. However, experts caution that while appetite suppression can facilitate weight loss, it does not address underlying emotional or psychological drivers of eating, which may resurface if medication is discontinued.
Citation
Stress and Obesity in Annual Reviews Publication
Conclusion
GLP-1 agonists like Ozempic/semaglutide represent a breakthrough in obesity management, offering substantial, sustained weight loss and cardiometabolic benefits supported by robust RCT evidence. While tirzepatide may edge in efficacy, semaglutide remains foundational. Ongoing research into combinations and orals promises further advances, but long-term adherence and real-world outcomes warrant monitoring.
Yet these drugs are not magic bullets. Without long-term strategies, ethical frameworks, and equitable access, they risk becoming another short-lived medical obsession rather than a sustainable public-health solution.
If you think this information is useful you can…
Get updates and read additional stories on the Health Orbit Fan Page.
For Guest posts, sponsored posts and other details, please click the ‘Contact Us’ page.




